|
Effects of Omega-3
on lipid profile and haematological parameters
in hyperlipidemic rats
......................................................................................................................................................................
Kawa Dizaye
Hozan Jarjees
Hawler Medical University, Erbil, Iraq
Correspondence:
Dr. Kawa Dizaye
Professor of Pharmacology
Hawler Medical University, Erbil, Iraq
Tel: 009647504452392
Email: dr_kawadizaye@yahoo.com
|
ABSTRACT
Background: There is good evidence
that omega-3 fatty acids found in fish oil
can help to prevent and treat atherosclerosis
by preventing the development of plaque
and blood clots. Omega-3 can also help prevent
heart disease, lower blood pressure, and
reduce the level of triglycerides in the
blood. The present study was designed to
evaluate and compare the effects of different
doses of omega-3, gemfibrozil and atorvastatin
on lipid profile and haematological parameters
in hyperlipidemic rats.
Methods: Forty eight rats were divided
into two groups. The first groups included
18 rats' they were subdivided into three
subgroups each of 6 rats. The first subgroup
served as a control. The second and third
subgroups received omega-3 (15 mg/kg) and
(30 mg/kg) orally (PO) daily respectively.
The second group included 30 rats and received
atherogenic diet throughout the treatment
period and served as hyperlipidemic rats.
The hyperlipidemic model rats were subdivided
into five subgroups of six rats each. The
first subgroup served as a positive control.
The second and third subgroups received
omega-3 (15 mg/kg) and (30 mg/kg) PO daily
respectively. The fourth and fifth subgroups
received gemfibrozil (3.5 mg/kg) PO daily
and atorvastatin (2 mg/kg) PO daily respectively.
At the end of treatment period of all these
groups, the rats were subjected to various
biochemical and hematological tests.
Results: After four weeks of therapy,
(30mg/kg) of omega-3 showed a significant
reduction in the level of triglyceride (TG),
total cholesterol (TC) and low density lipoprotein
(LDL-C) in control rats, whereas (15mg/kg)
omega-3 could only reduce the level of TC
and LDL-C significantly. Four weeks of daily
administration of both doses of omega-3
produced significant reduction in serum
(TC, TG and LDL-C) of hyperlipidemic rats.
However neither (15mg/kg) of omega-3 nor
omega-3 (30mg/kg) could increase the level
of high density lipoprotein HDL-C in the
treated and non-treated hyperlipidemic rats.
Both doses of omega-3 produced a significant
increase in the level of HB, RBC and MCH
in normal rats. The same doses of omega-3
showed a significant increase in the levels
of hemoglobin (HB), red blood cell (RBC),
hematocrit (HTC) and mean corpuscular hemoglobin
(MCH) in hyperlipidemic rats after 4 weeks
of therapy.
Following four weeks treatment with both
gemfibrozile and atorvastatin there was
a significant reduction in serum (TC, TG
and LDL-C) and a significant rise in serum
HDL-C in hyperlipidemic rats.
Conclusion: Omega-3 was effective
in controlling lipid profile especially
serum (TC, TG and LDL-C). No significant
differences were found between the effects
of both doses omega-3 and gemfibrozile or
atorvastatin on TC, TG, and LDL-C of hyperlipidemic
rats. In contrast to omega-3, gemfibrozile
and atorvastatin induced a significant raise
in the level of HDL-C. Omega-3 was effective
in increasing the levels of HB, RBC, HTC
and MCH in hyperlipidemic rats.
Key words: Omega 3, Gemfibrozile,
Atorvastatin, Lipid profiles, hyperlipidemic
rats
|
Hyperlipidemia is a lipid abnormality
with genetic or familial origins (primary hyperlipidemia).
Hyperlipidemia could also be caused by endocrine,
hepatic or renal diseases (secondary hyperlipidemia).
Primary hyperlipidemia includes familial or polygenic
hypercholesterolemia, familial combined hyperlipidemia,
familial hypertriglyceridemia, and dysbetalipoproteinemia
(1).
Concomitant elevation of circulating levels of
triglyceride-rich VLDL and cholesterol-rich LDL
is recognized as being associated with an increased
risk of premature coronary artery disease (2).
There is good evidence that omega-3 fatty acids
(namely EPA and DHA) found in fish oil can help
prevent and treat atherosclerosis by preventing
the development of plaque and blood clots. Omega-3
can also help prevent heart disease, lower blood
pressure, and reduce the level of triglycerides
(fats) in the blood. One preliminary study found
that people with high cholesterol who took fish
oil and red yeast rice lowered cholesterol levels
about as much as people who took simvastatin.
People with heart disease or those who need to
lower triglycerides may need to take fish oil
supplements (3) and are characteristic of subjects
who exhibit a lipid phenotype typical of combined
hyperlipidemia (4).
Atherosclerosis is a disease of large and medium-sized
muscular arteries characterized by inflammation
and dysfunction of the lining of the involved
blood vessels and the buildup of cholesterol and
lipids. This results in the formation of a plaque,
obstruction of blood flow and diminished oxygen
supply to target organs (5).
This dysfunction may arise due to many factors
like vessel injury and collagen exposure, metabolite
deposition in the vessel wall (increase in lipid,
cholesterol), or change in vascular reactivity
due to change in the rate or force with which
blood flows (6, 7).
The present study was designed to evaluate and
compare the effects of different doses of omega-3,
gemfibrozil and atorvastatin on lipid profile
and haematological parameters in hyperlipidemic
rats.
Animals
A total of 48 rats of both sexes were used in
the present study. Their weight ranged from (170-
250 grams) and they were aged 60 days, the rats
were obtained from Mousil and Abu ghreb. Once
received they were kept in the animal house in
the College of Medicine under controlled conditions
of a 12 hour light / 12 hour dark cycle in a room
temperature of 25 C°.
The rats were divided into two groups. The first
groups included 18 rats which received standard
diet throughout the experimental period and were
subdivided into three subgroups each of 6 rats.
The first subgroup served as a control. The second
subgroup received a daily single dose of omega-3
(15mg/kg) orally (PO). The third subgroup received
a daily double dose of omega-3 (30mg/kg) PO.
The second group included 30 rats and received
an atherogenic diet (79% standard diet + 21% Butter
fat) throughout the treatment period and served
as hyperlipidemic rats. The hyperlipidemic model
rats were subdivided into four subgroups, each
group having six rats. The first subgroup served
as a positive control. The second subgroup received
daily single dose of omega-3 (15mg/kg) PO. The
third subgroup received a daily double dose of
omega-3 (30mg/kg) PO. The fourth subgroup received
a daily single dose of gemfibrozil (3.5mg/kg)
PO, and the fifth subgroup received a daily single
dose of atorvastatin (2mg/kg) PO.
At the end of the treatment period, the animals
were subjected to various biochemical parameters
(biochemical and hematological parameters). The
animals were deprived of food overnight, anesthetized
using light chloroform and sacrificed by cervical
decapitation. Blood samples were collected from
the rats for determination of serum total cholesterol,
triglycerides, high density lipoprotein-C and
low density lipoprotein-C, besides some of hematological
parameters (HB, RBC, HTC, and MCH).
Statistical analysis
All data are expressed as means± standard
error means (M±SEM) and statistical analysis
was carried out using statistically available
software (SPSS Version 11.5). Data analysis was
made using one-way analysis of variance (ANOVA).
The comparison among groups was done using Duncan
test. P<0.05 was considered as statistical
significance.
Effects of omega-3 on lipid
profiles
Daily administration of omega-3 (30mg/kg) induced
a significant reduction in the level of TG in
normal rats. The level of triglyceride of normal
rats also decreased by (15mg/kg) of omega-3 but
the result turned out to be statistically non-significant
(Table 1).
Table 1: Effect of different doses of omega-3
(15mg/kg) and (30mg/kg) on the lipid profile of
normal rats (n=18)
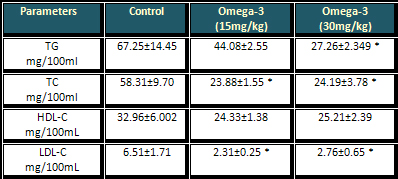
* (P<0.05) when compared to control group
Both doses of omega-3 (15mg/kg) and (30mg/kg)
have the same significant efficacy in reducing
the level of both TC and LDL-C of the normal rats,
whereas they have no significant effects on the
level of HDL-C of normal rats as shown in Table
1.
Effects of omega-3 on lipid profiles of hyperlipidemic
rats
There was a marked increase in the level of serum
triglyceride and TC and LDL-C in the animals treated
with atherogenic diet compared to the control
group indicating the induction of hyperlipidemia
as shown in Table 2.
Click here for Table
2: Effect of different doses of omega-3, gemfibrozile
and atorvastatin on the lipid profile of hyperlipidemic
rats (n=36)
Different letters indicate the
significance of the result (P<0.05).
Compared to the hyperlipidemic rat model both
doses of omega-3 (15mg/kg) and (30mg/kg) produced
significant reduction in the level of TG. Moreover
the same doses of omega-3 could decrease the level
of TC and LDL-C hyperlipidemic rats significantly.
Compared to the control group no significant changes
appeared in the level of HDL in the treated and
non-treated hyperlipidemic rats (Table 2).
Effects of omega-3 on some
haematological parameters
Both doses of omega-3 significantly increased
the level of HB, RBC and MCH of control rats,
while the same doses of omega 3 induced a non-significant
rise in the level of HTC as shown in Table 3.
Table
3: Effect of different doses of omega-3 on the
haematological parameters of normal rats

Rats fed with atherogenic diet for thirty days
displayed non-significant reduction in the levels
of HB, RBC and HTC, whereas it significantly reduced
MCH compared to the control group. Both doses
of omega-3 (15mg/kg) and (30mg/kg) significantly
increased the level of HB, RBC and HTC compared
with both normal and hyperlipidemic groups as
shown in Table 4.
Table 4: Effects of
different doses of omega-3 on the haematological
profiles of
hyperlipidemic rats
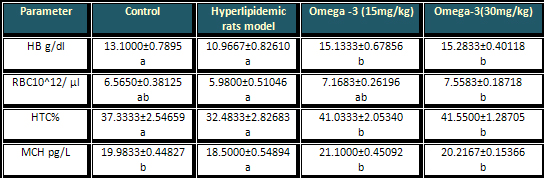
Compared to the control
group both doses of omega-3 showed no significant
changes in the level of MCH. However there was
a significant difference between the effects of
omega-3 with that of hyperlipidemic rats as shown
in Table 4.
Effects of gemfibrozil on
lipid profiles of hyperlipidemic rats:
Compared to the hyperlipidemic rat model gemfibrozil
produced significant reduction in the level of
TG, TC and LDL-C (Table 2).
No significant differences were found between
the effects of both doses omega-3 and gemfibrozil
on TG, total cholesterol and LDL of hyperlipidemic
rats. However gemfibrozil unlike omega-3 significantly
increased the level of HDL-C as shown in Table
2.
Effects of atorvastatin
on lipid profiles of hyperlipidemic rats:
Compared to the hyperlipidemic rat model atorvastatin
produced significant reduction in the level of
TG, total cholesterol and LDL (Table 2).
No significant differences were found between
the effects of both doses omega-3 and atorvastatin
on TG, total cholesterol and LDL of hyperlipidemic
rats however atorvastatin could significantly
increase the level of HDL as shown in Table 2.
According to the lipid hypothesis,
abnormally high cholesterol levels (hypercholesterolemia),
or more correctly, higher concentrations of LDL-C
and lower concentrations of functional HDL-C are
strongly associated with cardiovascular disease
because these promote atheroma development in
arteries (atherosclerosis). This disease process
leads to myocardial infarction (heart attack),
stroke and peripheral vascular disease. Since
higher blood concentrations of LDL-C, especially
the smaller and denser LDL particles, contribute
to this process, they are often termed "bad
cholesterol" because they have been linked
to atheroma formation, while high concentrations
of functional HDL-C, which can remove cholesterol
from cells and atheroma, offers protection (8).
Concomitant elevation of circulating levels of
triglyceride-rich VLDL and cholesterol-rich LDL
is recognized as being associated with an increased
risk of premature coronary artery disease (2)
and is characteristic of subjects who exhibit
a lipid phenotype typical of combined hyperlipidemia
(4).
In the present study, serum triglycerides were
significantly reduced in hyperlipidemic and normal
rats treated with omega-3 at the dose of (15mg/kg)
for a single dose and (30mg/kg) for a double dose
after 4 weeks of treatment. This result is in
agreement with another study by Harris et al (1983)
who found that omega-3 significantly reduced serum
triglycerides in hypertriglyceridemic patients
by 25 % to 35 % after 12 weeks of therapy (9).
Similar findings were reported by Sanders and
Hochland (1983), Negakawa et al. (1983) and Zucker
et al. (1988) (10, 11, 12) who found that fish
oil (< 20 g/d) induced a marked decrease in
triglyceride concentration in hyperlipidemic patients.
This antitriglyceridemic effect of omega-3 on
hyperlipidemic rats is in consensus with Simopoulos
(1991) and Thomas et al. (2000) who observed that
triglyceride concentration was reduced considerably
by omega-3 in patients with hypertriglyceridemia
(13, 14).
The mechanism responsible for the triglyceride-lowering
effect of omega-3 is poorly defined. In theory
it could be related to decreased VLDL-C production
(presumably secondary to decreased availability
of hepatic free cholesterol for particle assembly),
increased clearance of VLDL-C through the LDL
receptor (or other lipoprotein receptors), increased
delipidation of VLDL particles via LPL, or a combination
of the above mechanisms (15).
In this study, the level of total cholesterol
was significantly reduced in hyperlipidemic rats
treated with both doses of omega-3 after 4 weeks
of treatment. This finding is in agreement with
Kobatake et al. (1984) who observed that omega-3
significantly reduced serum total cholesterol
after 20 days of therapy in hyperlipidemic subjects
(16). Whereas Harris (1997) found that a large
dose of omega-3 (4 g per day) has no significant
effect on the level of total cholesterol in hyperlipidemic
subjects after 2 weeks of treatment so this difference
might be due to the short term treatment with
omega-3 (17).
In the present study, hyperlipidemic rats treated
with omega-3 at the doses of (15mg/kg) and (30mg/kg)
showed no significant increase in the level of
HDL after 4 weeks of treatment. This result is
incompatible with another study by (Mori 2000)
who found that HDL-C concentration was increased
significantly in hyperlipidemic subjects (18).
Furthermore Harris (1997) concluded that 4 g per
day of omega-3 increased HDL-C cholesterol levels
by 1 to 3 percent after 4 weeks of treatment.
This effect of omega-3 could be due to the fact
that omega-3 significantly reduced total cholesterol
in hyperlipidemic and normal rats (19).
LDL-C was significantly reduced in hyperlipidemic
rats treated with both doses of omega-3 after
4 weeks of treatment. This is incompatible with
the observation of Mori (2000) who observed that
there was usually no significant changes in LDL-cholesterol
concentration associated with omega-3 administration
in hyperlipidemic subjects (18). On the contrary,
especially with high doses of omega-3 FAs used
in the treatment of hypertriglyceridemia, LDL
levels may rise by 10 %, this effect being even
more pronounced in patients with extreme TG elevations
at baseline (17. 19).
In another investigation Sanders and Hochland
(1983) reported that there were modest decreases
in LDL concentration for the normal subjects who
received (< 20 g/d) of fish oil after 4 weeks
of treatment (10), similar findings were reported
by Negakawa et al (1983) and Zucker et al (1988)
(11, 12).
In the present study, HB and RBC were noticeably
increased in hyperlipidemic rats treated with
omega-3 at the dose of (15mg/kg) and (30mg/kg).
These results are compatible with another study
by Abbas et al (2009) who found that administration
of omega-3 was associated with an increase in
the levels of HB and RBC in sucrose treated rats
(20).
In this study, HTC was significantly increased
in hyperlipidemic rats treated with omega-3. This
result is incompatible with another study by Ghaderpanahi
et al (2010) who found that administration of
1g of fish oil in elderly subjects has no significant
effects on the level of HTC (21).
In this research, MCH was increased significantly
in hyperlipidemic rats treated with omega-3 (15mg/kg)
and (30mg/kg). This result was replicated in another
study by Nwabueze et al (2011) who found that
(MCH) was significantly (P<0.05) higher in
Heterobranchus bidorsalis fish fed on feeds containing
2000mg and 1000mg omega-3 than in control fish
(22).
Gemfibrozil treatment produced a significant reduction
in the serum triglyceride of hyperlipidemic rats
similar to that of omega-3. This result was quite
similar to that reported by Keijiro Saku et al
(1985) who found that gemfibrozil significantly
reduced serum triglycerides by 46 % after 12 weeks
of therapy in hyperlipidemic patients (23).
Moreover it is accordance with the result of Irish
and Thompson (1996) who detected that gemfibrozil
lowered serum triglycerides by 44% after 6 weeks
of therapy in hyperlipidemic patients (2).
The result of this study showed that total cholesterol
was significantly reduced in hyperlipidemic rats
treated with gemfibrozil after 4 weeks of treatment.
This result is in agreement with another study
by Keijiro Saku et al (1985) who found that gemfibrozil
significantly reduced total cholesterol by 47%
after 12 weeks of therapy in hyperlipidemic patients
(23).
In this study, HDL was significantly increased
in hyperlipidemic rats treated with gemfibrozil
after 4 weeks of treatment. This result is in
consensus with studies by Irish and Thompson (1996)
who concluded that gemfibrozil significantly increased
HDL by 36% and 31% respectively after 12 weeks
of therapy in hyperlipidemic patients (2, 24).
In the present study serum LDL was reduced in
hyperlipidemic rats treated with gemfibrozil after
4 weeks of treatment. This result is in agreement
with another study by Manninen et al (1982) who
found that gemfibrozil significantly reduced LDL
by 23% after 12 weeks of therapy in hyperlipidemic
patients (25), whereas Irish and Thompson (1996)
reported that gemfibrozil has no significant effect
on the level of LDL in hyperlipidemic patients
even after 12 weeks of treatment. Therefore this
result is incompatible with the finding of this
study (24).
In this study, serum TG was significantly reduced
in hyperlipidemic rats treated with atorvastatin
after 4 weeks of treatment. This result is in
agreement with studies of Athyros et al (2002)
and Branchi et al (1999) who found that atorvastatin
20 mg daily dose significantly reduced TG by 31%,
20 % respectively in hyperlipidemic patients after
2 months of therapy (26, 27). In accordance with
reports of Stein et al (1998) the effect of atorvastatin
on serum TG was largely dependent on the baseline
serum triglyceride level and, in patients with
low serum triglyceride; there was little if any
hypotriglyceridemic response (28). The relationship
of the hypotriglyceridemic activity to the baseline
serum triglyceride level may explain why some
authors found only small effects of statins on
serum triglycerides, whereas others reported greater
lowering effects.
The differences in the hypotriglyceridemic response
among the studies are likely to be due to differences
in the patient populations. It is generally accepted
that HMG CoA reductase does not play a direct
role in the repletion of TG levels. Atorvastatin
administration, however, produces marked reduction
in TG levels in hyperlipidemic patients (29).
In the present study, atorvastatin as gemfibrozil
and omega-3 significantly reduced the level of
total cholesterol in hyperlipidemic rats after
4 weeks of treatment. These results are in agreement
with results of studies conducted by Nawrocki
et al (1995) and Marian et al (2006) who found
that atorvastatin reduced plasma cholesterol up
to 45% in patients with primary hypercholesterolemia
(30, 31).
In this research, LDL-C was significantly reduced
in hyperlipidemic rats treated with atorvastatin
after 4 weeks of treatment. This is in accordance
with the observations of Hing-Chung et al (2006)
who found that atorvastatin 20 mg daily for 12
weeks of treatment significantly decreased LDL-C
in comparison with 10 and 40 mg of atorvastatin
in hyperlipidemic patients (32).
This reduction of serum cholesterol could be due
to inhibition of HMG-CoA reductase which catalyzes
the conversion of HMG-CoA to mevalonate which
decreases the cholesterol synthesis (33, 34).
In the present study, HDL-C was significantly
increased in hyperlipidemic rats treated with
atorvastatin after 4 weeks of treatment. This
result was quite similar to that reported by Jeevan
et al, (2008) who found that atorvastatin significantly
increased HDL-C for 12 weeks of treatment (35).
The mechanism underlying the increase in HDL-C
levels observed during statin therapy is poorly
understood. Available evidence suggested that
increase in HDL-C with statin therapy results
from a combination of increased expression of
apoA-I and reduced HDL remodeling as a consequence
of lowering triglyceride levels (35, 36).
There is also evidence that increases in HDL-C
during statin therapy may be related to the decrease
in the activity of cholesteryl ester transfer
protein, likely due to depletion of levels of
very low-density lipoprotein and LDL particles
(37).
No significant differences were found between
the effects of both doses of omega-3 with gemfibrozil
and atorvastatin on TG, total cholesterol and
LDL-C of hyperlipidemic rats, however gemfibrozil
and atorvastatin unlike omega-3, significantly
increased the level of HDL-C.
1- Omega-3 was efficient
in reducing serum TC, TG and LDL-C. However it
was not effective in significantly altering serum
HDL-C in hyperlipidemic rats.
2- Omega-3 was effective in increasing
the levels of HB, RBC, HTC, and MCH in hyperlipidemic
rats.
3- No significant differences were found
between the effects of both doses of omega-3 and
gemfibrozile or atorvastatin on TG, TC and LDL-C
of hyperlipidemic rats. In contrast to omega-3,
gemfibrozile and atorvastatin induced a significant
rise in the level of HDL-C.
1- Farnier M and Davignon J. Current and future
treatment of hyperlipidemia: the role of statins.
Am J Cardiol ; 82 (4B). 1998; 3J-10J.
2- Thompson GR. A Handbook of Hyperlipidemia. London,
England: Current Science. 1990; 69-85, 177-194.
3- Mita T, Watada H, Ogihara T, Nomiyama T, Ogawa
O, et al. "Eicosapentaenoic acid reduces the
progression of carotid intima-media thickness in
patients with type 2 diabetes". Atherosclerosis.
2007; 191 (1): 162-167.
4- Arad Y, Ramakrishnan R and Ginsberg H. Lovastatin
therapy reduces low density lipoprotein apo B level
in subjects with combined hyperlipidemia by reducing
the production of apo B containing lipoproteins:
implications for the pathophysiology of apo B production.
J Lipid Res. 1990; 31:567-582.
5- Anderson TJ. Assessment and treatment of endothelial
dysfunction in humans. J Am Coll Cardiol. 1999;
34 (3): 631-8.
6- Papaioannou TG, Karatzis EN, Vavuranakis M, Lekakis
JP and Stefanadis C. Assessment of vascular wall
shear stress and implications for atherosclerotic
disease. Int J Cardiol. 2006; 113(1):12-18.
7- Pantos J, Efstathopoulos E and Katritsis DG.
Vascular wall shear stress in clinical practice.
Curr Vasc Pharmacol. 2007; 5(2):113-119.
8- Durrington P. Dyslipidaemia. Lancet. 2003; 362
(9385): 717-731.
9- Harris WS. Fish oils and plasma lipid and lipoprotein
metabolism in human: a critical review. J lipid
Res. 1989; 30:785-807.
10- Sanders TAB and Hochland MC. A comparison of
the influence on plasma lipids and platelet function
of supplements of omega-3 and omega-6 polyunsaturated
fatty acids. Br J Nutr. 1983; 50:521-9.
11- Negakawa Y, Orimo H, Harasawa M, Morita I ,Yashiro
K et al. Effect of EPA on platelet aggregation and
composition of fatty acids in man. Atherosclerosis.
1988; 47:71-5.
12- Zucker ML, Bilyeu D, Helmkamp GM, Harris WS
and Dujovne CA. Effects of dietary fish oil on platelet
function and plasma lipids in hyperlipoproteinemic
and normal subjects. Atherosclerosis. 1988; 73:
pp13-22.
13-Simopoulos AP. Omega-3 fatty acids in health
and disease and in growth and development. Am J
Clin Nutr. 1991; 54 (3): 438-63.
14- Thomas TR, Fischer BA, Kist WB, Horner KE and
Cox RH. Effects of exercise and fatty acids on postprandial
lipemia. J Appl Physiol. 2000; 88:2199-2204.
15- William L. Isley,1, John M. Miles, Bruce W.
Patterson, and William S. Harri. The effect of high-dose
simvastatin on triglyceride-rich lipoprotein metabolism
in patients with type 2 diabetes mellitus. Journal
of Lipid Research. 2006; Volume 47,
16-Kobatake Y, Kuroda K, Jinnouchi H, Nishide E
and Innami S. Differential effects of dietary eicosapentaenoic
and docosahexaenoic fatty acids on lowering of triglyceride
and cholesterol levels in the serum of rats on hypercholesterolemic
diet. J Nutr Sci Vitaminol. 1984; 30(4):357-72.
17- Harris WS. n-3 fatty acids and serum lipoproteins:
human studies. Am J Clin Nutr. 1997; 65: 1645s-1654s.
18-Mori TA, Burke V, Puddey IB, Watts GF, ONeal
DN et al. Purified eicosapentaenoic and docosahexaenoic
acids have differential effects on serum lipids
and lipoproteins, LDL particle size, glucose, and
insulin in mildly hyperlipidemic men. Am J Clin
Nutr. 2000; 71: 1085-1094.
19- Harris WS, Ginsberg HN, Arunakul N, Shachter
NS, Windsor SL et al. Safety and efficacy of Omacor
in severe hypertriglyceridemia. J Cardiovas Risk.
1997; 4: 385-391.
20-Abbas B. Qadir, Ismail M. Maulood and Zana R.
Majeed. Effects of omega-3 and L-carnitine on some
hematological parameters in sucrose treated male
albino rats: J. Duhok Univ; 2009; 12 (1) Pp 125-128.
21- Ghaderpanahi M, Fakhrzadeh H, Sharifi F, Mirarefin
M, Badamchizade Z et al. The Effects of Fish Oil
Supplementation on Hematologic Pattern of the Elderly
"Kahrizak Elderly Study" tums journal.
2010; (9) :21.
22- Nwabueze A. A., Nwabueze E. O. and Mayor E.
P: Effects of Omega-3 Fatty Acids in Fish Feeds
on Haematological Profile of Heterobranchus bidorsalis:
J. Agric. Food. Tech. 2011; 1(3) 26- 30.
23- Keijiro Saku, Peter S. Gartside, Barbara A.
Hynd, and Moti L. Kashyap. Mechanism of Action of
Gemfibrozil on Lipoprotein Metabolism: J Clin Invest.
1985; 75(5): 1702-1712.
24-Irish AB and Thompson CH. The effects of gemfibrozil
upon the hypercoagulable state in dyslipidaemic
patients with chronic renal failure. Nephrol Dial
Transplant. 1996;11(11):2223-8.
25- Manninen V, Malkonen M., Eisalo A, Virtamo J,
Tuomilehto J et al (1982). Gemfibrozil in the treatment
of dyslipidaemia. A 5-year follow-up study. Acta.
Med. Scand. 668(Suppl.). 1982; 82- 87.
26- Athyros VG, Giouleme OI, Nikolaidis NL, Vasiliadis
TV, Bouloukos VI et al. Long-term follow up of patients
with acute hypertriglyceridemia-induced pancreatitis.
J Clin Gastroenterol. 2002; 34:472-477.
27-Branchi A, Fiorenza AM, Rovellini A, Torri A,
Muzio F et al. Lowering effects of four different
statins on serum triglyceride level. Eur J Clin
Pharmacol. 1999; 55(7): 499-502.
28- Stein EA, Lane M and Laskarzewski P. Comparison
of statins in hypertriglyceridemia. Am J Cardiol.
1998; 81: 66-69.
29- Ginsberg HN. Effects of statins on triglyceride
rnetabolism. Am J Cardiol. 1998; 8 1-44 32B35B.
30- Nawrocki JW, Weiss SR, Davidson MH Sprecher
DL and Schwartz SL. Reduction of LDL cholesterol
by 25% to 60% in patients with primary hypercholesterolemia
by atorvastatin, a new HMG-CoA reductase inhibitor.
Arterioscler Thromb Vasc Biol. 1995; 15:678-682.
31- Marian G, Soledad GV and Vicente L. Effects
of Atorvastatin on inflammatory and fibrinolytic
parameters in patients with chronic kidney disease.
J Am Soc Nephrol. 2006; 17: S231-S235.
32-Hing-Chung L, Chih-Hsun C, Mei-Chih W Hsiu-Man
Keng, Chih-Chen Lu et al. The Effects of Different
Doses of Atorvastatin on Plasma Endothelin-1 Levels
in Type 2 Diabetic Patients with Dyslipidemia. Exp
Biol Med. 2006; 231:1010-1015.
33-Williams D and Feely J. Pharmacokinetic-pharmacodynamic
drug interactions with HMG-CoA reductase inhibitors.
Clin Pharmacokinet. 2002; 41:343-70.
34- Schachter M (2005). Chemical, pharmacokinetic
and pharmacodynamic properties of statins: an update.
Fundam Clin Pharmacol. 2005; 19:117-125.
35- Jeevan k.S, Mungli P and Sudashna T. Effect
of atorvastatin paraoxonase activity in patients
with hyperlipidemia. J Biochem. 2008; 1815-1823.
36- Martin G, Duez H, Blanquart C, Berezowski V,
Poulain P, Fruchart JC et al. Statin-induced inhibition
of the Rho-signaling pathway activates PPAR alpha
and induces HDL apoA-I. J Clin Invest. 2001; 107:1423-1432.
37- Guerin M, Lassel TS, Le Goff W, Van Tol A, Steiner
G et al. Action of atorvastatin in combined hyperlipidemia:
preferential reduction of cholesteryl ester transfer
from HDL to VLDL1 particles. Arterioscler Thromb
Vasc Biol. 2000; 20:189-197. |

